Abstract
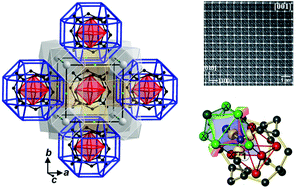
The compounds MNi21B20 (M = In, Sn) have been synthesized and their cubic crystal structure determined (space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, lattice parameters a = 7.1730(1) Å and a = 7.1834(1) Å, respectively). The structure can be described as a hierarchical partitioning of space based on a reo-e net formed by Ni3 species with large cubical, cuboctahedral and rhombicuboctahedral voids being filled according to [Ni1@Ni38], [M@Ni312], and [Ni26@B20@Ni324], respectively. The [Ni6@B20] motif inside the rhombicuboctahedral voids features an empty [Ni6] octahedron surrounded by a [B20] cage recently described in E2Ni21B20 (E = Zn, Ga). Position-space bonding analysis using ELI-D and QTAIM space partitioning as well as 2- and 3-center delocalization indices gives strong support to an alternative chemical description of space partitioning based on face-condensed [B@Ni6] trigonal prisms as basic building blocks. The shortest B–B contacts display locally nested 3-center B–B–Ni bonding inside each trigonal prism. This clearly rules out the notion of [Ni6@B20] clusters and leads to the arrangement of 20 face-condensed [B@Ni23Ni33] trigonal prisms resulting in a triple-shell like situation Ni26@B20@Ni324(reo-e), where the shells display comparable intra- and inter-shell bonding. Both compounds are Pauli paramagnets displaying metallic conductivity.
m, lattice parameters a = 7.1730(1) Å and a = 7.1834(1) Å, respectively). The structure can be described as a hierarchical partitioning of space based on a reo-e net formed by Ni3 species with large cubical, cuboctahedral and rhombicuboctahedral voids being filled according to [Ni1@Ni38], [M@Ni312], and [Ni26@B20@Ni324], respectively. The [Ni6@B20] motif inside the rhombicuboctahedral voids features an empty [Ni6] octahedron surrounded by a [B20] cage recently described in E2Ni21B20 (E = Zn, Ga). Position-space bonding analysis using ELI-D and QTAIM space partitioning as well as 2- and 3-center delocalization indices gives strong support to an alternative chemical description of space partitioning based on face-condensed [B@Ni6] trigonal prisms as basic building blocks. The shortest B–B contacts display locally nested 3-center B–B–Ni bonding inside each trigonal prism. This clearly rules out the notion of [Ni6@B20] clusters and leads to the arrangement of 20 face-condensed [B@Ni23Ni33] trigonal prisms resulting in a triple-shell like situation Ni26@B20@Ni324(reo-e), where the shells display comparable intra- and inter-shell bonding. Both compounds are Pauli paramagnets displaying metallic conductivity.


 Please wait while we load your content...
Please wait while we load your content...