On the capacitance of narrow nanotubes
Abstract
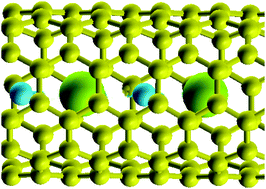
The storage of ions in narrow nanotubes is investigated by grand-canonical Monte Carlo simulations. The interaction between the ions is screened by the image charge on the wall of the tube, but at close distances it is still much larger than the thermal energy. Depending on the electrochemical potential imposed by the contact with an electrolyte solution, two different regimes can be distinguished at the potential of zero charge: for low values corresponding to an ionophobic pore the tube is almost empty; for high values – ionophilic pore – a one dimensional salt is formed. The two regions are separated by a narrow transition zone marked by strong fluctuations. Depending on the regime and on the value assumed for the dielectric constant, the interfacial capacity shows four, two, or in rare cases three maxima. The results are compared to a reference system of non-interacting ions, and discussed with respect to recent calculations within classical density functional theory.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...