Highly selective CO2vs. N2 adsorption in the cavity of a molecular coordination cage†
Abstract
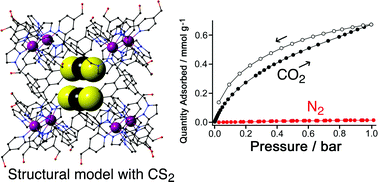
Two M8L12 cubic coordination cages, as desolvated crystalline powders, preferentially adsorb CO2 over N2 with ideal selectivity CO2/N2 constants of 49 and 30 at 298 K. A binding site for CO2 is suggested by crystallographic location of CS2 within the cage cavity at an electropositive hydrogen-bond donor site, potentially explaining the high CO2/N2 selectivity compared to other materials with this level of porosity.


 Please wait while we load your content...
Please wait while we load your content...