Ni(ii)-catalyzed asymmetric addition of arylboronic acids to cyclic imines†
Abstract
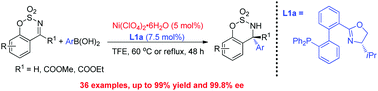
A Ni(II)-catalyzed asymmetric addition of arylboronic acids to cyclic aldimines and ketimines is reported. Our tropos phosphine-oxazoline biphenyl ligand is crucial for the high catalytic activity, which coordinates to Ni(II) to form a complex with a single axial configuration. The desired chiral amine products could be prepared with excellent yields (up to 99%) and enantioselectivities (up to 99.8%) under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...