Microfluidically fabricated pH-responsive anionic amphiphilic microgels for drug release
Abstract
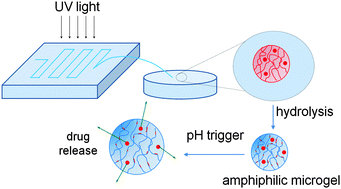
Amphiphilic microgels of different composition based on the hydrophilic, pH-responsive acrylic acid (AA) and the hydrophobic, non-ionic n-butyl acrylate (BuA) were synthesised using a lab-on-a-chip device. Hydrophobic droplets were generated via a microfluidic platform that contained a protected form of AA, BuA, the hydrophobic crosslinker, ethylene glycol dimethacrylate (EGDMA), and a free radical initiator in an organic solvent. These hydrophobic droplets were photopolymerised within the microfluidic channels and subsequently hydrolysed, enabling an integrated platform for the rapid, automated, and in situ production of anionic amphiphilic microgels. The amphiphilic microgels did not feature the conventional core–shell structure but were instead based on random amphiphilic copolymers of AA and BuA and hydrophobic crosslinks. Due to their amphiphilic nature they were able to encapsulate and deliver both hydrophobic and hydrophilic moieties. The model drug delivery and the swelling ability of the microgels were influenced by the pH of the surrounding aqueous solution and the hydrophobic content of the microgels.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...