Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms†
Abstract
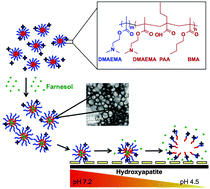
We previously reported on cationic, pH-responsive p(DMAEMA)-b-p(DMAEMA-co-BMA-co-PAA) block copolymer micelles with high affinity for dental and biofilm surfaces and efficient anti-bacterial drug release in response to acidic pH, characteristic of cariogenic (tooth-decay causing) biofilm microenvironments. Here, we show that micelle pH-responsive behaviors can be enhanced through alterations in corona : core molecular weight ratios (CCR). Although similarly stable at physiological pH, upon exposure to acidic pH, micelles with CCR of 4.1 exhibited more robust drug release than other CCR examined. Specifically, a ∼1.5-fold increase in critical micelle concentration (CMC) and ∼50% decrease in micelle diameters were observed for micelles with CCR of 4.1, compared to no changes in micelles with CCR of 0.8. While high CCR was shown to enhance pH-responsive drug release, it did not alter drug loading and dental surface binding of micelles. Diblocks were shown to encapsulate the antibacterial drug, farnesol, at maximal loading capacities of up to ∼27 wt% and at >94% efficiencies, independent of CCR or core size, resulting in micelle diameter increases due to contributions of drug volume. Additionally, micelles with small diameters (∼17 nm) show high binding capacity to hydroxyapatite and dental pellicle emulating surfaces based on Langmuir fit analyses of binding data. Finally, micelles with high CCR that have enhanced pH-responsive drug release and binding were shown to exhibit greater antibiofilm efficacy in situ. Overall, these data demonstrate how factors essential for nanoparticle carrier (NPC)-mediated drug delivery can be enhanced via modification of diblock characteristics, resulting in greater antibiofilm efficacy in situ.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...