Efficient water oxidation kinetics and enhanced electron transport in Li-doped TiO2 nanotube photoanodes†
Abstract
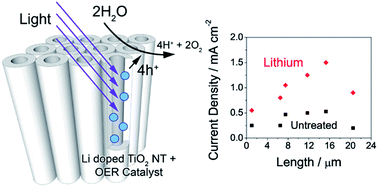
TiO2 nanotubes are a promising system to implement photoelectrochemical water splitting for the production of hydrogen fuel from sunlight. Besides the limited absorption spectrum, their performance is limited by the presence of trap states, which facilitate recombination, and the sluggish kinetics of the water splitting reaction. Long TiO2 nanotubes can potentially capture more light, but photogenerated charges are more prone to being lost to recombination as they must travel a longer path length. We demonstrate here that the use of Li doping extends the optimum nanotube length from 7 to 15 microns, yielding a photocurrent of 1.5 mA cm−2 under simulated sunlight at 1.65 VRHE. To identify the conditions under which photocurrent conversion is limited by the kinetics of the oxygen evolution reaction (OER), the OER efficiency of TiO2 nanotubes was quantified by measuring photocurrent in the presence of a hole scavenger. TiO2 nanotubes doped with Li exhibited up to 78% efficiency towards water splitting under an anodic bias above 1.2 VRHE. Under conditions where water oxidation was a limiting factor, addition of a CoOx OER catalyst yielded up to a twofold improvement in the photocurrent under a small applied bias of 0.4 VRHE.

 Please wait while we load your content...
Please wait while we load your content...