Electronic and optical properties of single crystal SnS2: an earth-abundant disulfide photocatalyst†
Abstract
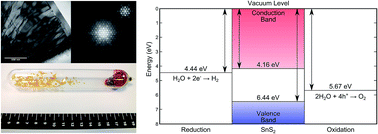
Tin disulfide is attractive as a potential visible-light photocatalyst because its elemental components are cheap, abundant and environmentally benign. As a 2-dimensional semiconductor, SnS2 can undergo exfoliation to form atomic layer sheets that provide high surface areas of photoactive material. In order to facilitate the deployment of this exciting material in industrial processes and electrolytic cells, single crystals of phase pure SnS2 are synthesised and analysed with modern spectroscopic techniques to ascertain the values of relevant semiconductor properties. An electron affinity of 4.16 eV, ionisation potential of 6.44 eV and work function of 4.81 eV are found. The temperature dependent band gap is also reported for this material for the first time. We confirm the valence band is formed predominately by a mixture S 3p and Sn 5s, while the conduction band consists of a mixture of Sn 5s and 5p orbitals and comment on the agreement between experiment and theory for values of band gaps.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry A Most Accessed Manuscripts

 Please wait while we load your content...
Please wait while we load your content...