Face and edge directed self-assembly of Pd12 tetrahedral nano-cages and their self-sorting†
Abstract
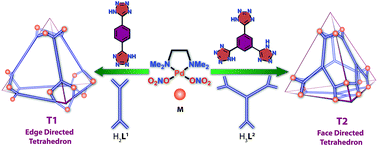
Reactions of a cis-blocked Pd(II) 90° acceptor [cis-(tmeda)Pd(NO3)2] (M) with 1,4-di(1H-tetrazol-5-yl)benzene (H2L1) and [1,3,5-tri(1H-tetrazol-5-yl)benzene] (H3L2) in 1 : 1 and 3 : 2 molar ratios respectively, yielded soft metallogels G1 and G2 [tmeda = N,N,N′,N′-tetramethylethane-1,2-diamine]. Post-metalation of the gels G1 and G2 with M yielded highly water-soluble edge and face directed self-assembled Pd12 tetrahedral nano-cages T1 and T2, respectively. Such facile conversion of Pd(II) gels to discrete tetrahedral metallocages is unprecedented. Moreover, distinct self-sorting of these two tetrahedral cages of similar sizes was observed in the self-assembly of M with a mixture of H2L1 and H3L2 in aqueous medium. The edge directed tetrahedral cage (T1) was successfully used to perform Michael reactions of a series of water insoluble nitro-olefins assisted by encapsulation into the cage in aqueous medium.

 Please wait while we load your content...
Please wait while we load your content...