The reactivity and conformational control of cyclic tetrapeptides derived from aziridine-containing amino acids†
Abstract
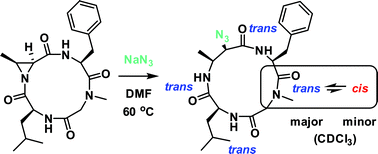
Among the smallest of the macrocyclic peptides, 12- and 13-membered cyclic tetrapeptides are particularly noteworthy because they exhibit a broad spectrum of biological activities due to their innate capacity to mimic β-turns in proteins. In this report, we demonstrate that aziridine-containing cyclic tetrapeptides offer a platform to interrogate the conformational properties of tetrapeptides. We show that aziridine ring-opening of 12-membered cyclic tetrapeptides yields exclusively 13-membered α3β macrocycles, regardless of peptide sequence, nucleophile, aziridine β-carbon substitution, or stereochemistry. NMR and computational studies on two related aziridine-containing cyclic tetrapeptides revealed that the amide conformations of their N-acyl aziridines are similar, and are likely the determinant of the observed ring-opening regioselectivity. Interestingly, some of the resulting 13-membered α3β macrocycles were found to be conformationally heterogeneous. This study on the reactivity and conformational control of aziridine-containing cyclic tetrapeptides provides useful insight on the design and development of macrocyclic therapeutics.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...