Ferrocenyl chiral bisphosphorus ligands for highly enantioselective asymmetric hydrogenation via noncovalent ion pair interaction†
Abstract
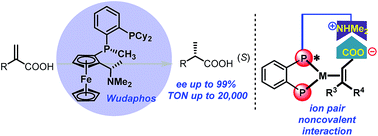
A new class of ferrocenyl chiral bisphosphorus ligand, Wudaphos, was developed, and exhibits excellent ee and activity (ee up to 99%, TON up to 20 000) for the asymmetric hydrogenation of both 2-aryl and 2-alkyl acrylic acids through ion pair noncovalent interaction under base free and mild reaction conditions. Well-known anti-inflammatory drugs such as naproxen and ibuprofen together with the intermediate for the preparation of Roche ester and some bioactive compounds were also efficiently obtained with excellent ee. Control experiments were conducted and revealed that the ion pair noncovalent interaction and chain length played important roles.

 Please wait while we load your content...
Please wait while we load your content...