Asymmetric synthesis of allylic amines via hydroamination of allenes with benzophenone imine†
Abstract
Rhodium-catalyzed highly regio- and enantioselective hydroamination of allenes is reported. Exclusive branched selectivities and excellent enantioselectivities were achieved applying a rhodium(I)/Josiphos catalyst. This method permits the practical synthesis of valuable α-chiral allylic amines using benzophenone imine as ammonia carrier.
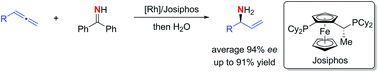

 Please wait while we load your content...
Please wait while we load your content...