Peptidines: glycine-amidine-based oligomers for solution- and solid-phase synthesis†
Abstract
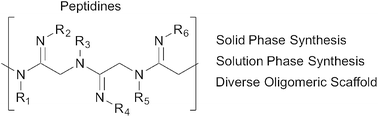
Efforts to emulate biological oligomers have given rise to a host of useful technologies, ranging from solid-phase peptide and nucleic acid synthesis to various peptidomimetic platforms. Herein we introduce a novel class of peptide-like oligomers called “peptidines” wherein each carbonyl O-atom within poly-N-alkyl glycine oligomers is replaced with a functionalized N-atom. Compared to peptoids or peptides, the presence of this amidine N-substituent in peptidines effectively doubles the number of diversification sites per monomeric unit, and can decrease their overall conformational flexibility. We have developed iterative solution- and solid-phase protocols for the straightforward assembly of peptidines containing diverse backbone and amidine substituents, derived from readily available primary and secondary amines. We have also performed crystallographic and computational studies, which demonstrate a strong preference for the trans (E) amidine geometry. Given their straightforward synthetic preparation and high functional group density, peptidines have the potential to serve as useful tools for library generation, peptide mimicry, and the identification of biologically active small molecules.

 Please wait while we load your content...
Please wait while we load your content...