Synthesis and activity of a diselenide bond mimetic of the antimicrobial protein caenopore-5†
Abstract
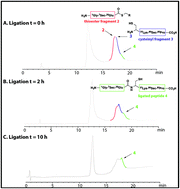
Antimicrobial proteins are a rich source of new lead compounds for the development of new drugs that will tackle global resistance towards existing antibiotics. Caenopore-5 (Cp-5) is an antimicrobial protein (AMP) expressed in the intestine of the nematode Caenorhabditis elegans and is a member of the lipid binding saposin-like-protein family, composed of 5 α-helices and 3 disulfide bonds. Substitution of the 7Cys and 81Cys by two selenocysteine 7U and 81U afforded a selenocysteine analogue [7Sec-81Sec]-Cp-5, which displayed a higher stability (using thermal circular dichroism) compared to the native protein Cp-5. [7Sec-81Sec]-Cp-5 and an N-terminal truncated peptide exhibited cell permeability similar to the wild type Cp-5.

 Please wait while we load your content...
Please wait while we load your content...