Accelerating chemoselective peptide bond formation using bis(2-selenylethyl)amido peptide selenoester surrogates†
Abstract
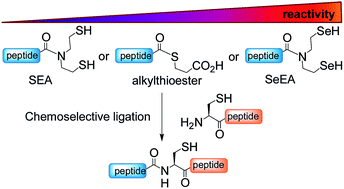
Given the potential of peptide selenoesters for protein total synthesis and the paucity of methods for the synthesis of these sensitive peptide derivatives, we sought to explore the usefulness of the bis(2-selenylethyl)amido (SeEA) group, i.e. the selenium analog of the bis(2-sulfanylethyl)amido (SEA) group, for accelerating peptide bond formation. A chemoselective exchange process operating in water was devised for converting SEA peptides into the SeEA ones. Kinetic studies show that SeEA ligation, which relies on an initial N,Se-acyl shift process, proceeds significantly faster than SEA ligation. This property enabled the design of a kinetically controlled three peptide segment assembly process based on the sequential use of SeEA and SEA ligation reactions. The method was validated by the total synthesis of hepatocyte growth factor K1 (85 AA) and biotinylated NK1 (180 AA) domains.


 Please wait while we load your content...
Please wait while we load your content...