Hydrogenation of the liquid organic hydrogen carrier compound dibenzyltoluene – reaction pathway determination by 1H NMR spectroscopy†
Abstract
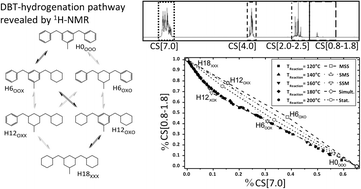
The catalytic hydrogenation of the LOHC compound dibenzyltoluene (H0-DBT) was investigated by 1H NMR spectroscopy in order to elucidate the reaction pathway of its charging process with hydrogen in the context of future hydrogen storage applications. Five different reaction pathways during H0-DBT hydrogenation were considered including middle-ring preference (middle-side-side, MSS), side-middle-side order of hydrogenation (SMS), side-ring preference (SSM), simultaneous hydrogenation of all three rings without intermediate formation and statistical hydrogenation without any ring preference. Detailed analysis of the 1H NMR spectra of the H0-DBT hydrogenation over time revealed that the reaction proceeds with a very high preference for the SSM order at temperatures between 120 °C and 200 °C and 50 bar in the presence of a Ru/Al2O3-catalyst. HPLC analysis supported this interpretation by confirming an accumulation of H12-DBT species prior to full hydrogenation to H18-DBT with middle ring hydrogenation as the final step.


 Please wait while we load your content...
Please wait while we load your content...