Reactive crystallization of β-lactam antibiotics: strategies to enhance productivity and purity of ampicillin
Abstract
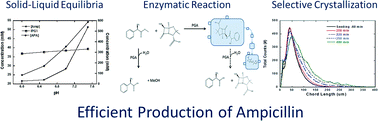
Seeded reactive crystallization in the manufacture of semi-synthetic β-lactam antibiotics is described and the beneficial effects on yield are discussed. Conventional enzymatic synthesis of β-lactam is limited by secondary hydrolysis reactions that consume the desired product as it is being produced. Recent work in this area has pointed to the potential advantage of performing reactions at conditions that allow product crystallization to reduce the rate of secondary hydrolysis by protecting ampicillin in the solid phase. However, these approaches led to crystallization of both D-phenylglycine and ampicillin, which will greatly increase downstream processing. In the work described here, seeded crystallization is used to promote secondary nucleation of the desired ampicillin while it is being produced by the synthesis reaction, thereby selectively crystallizing ampicillin. Quantification of the solid phase confirmed selective crystallization of ampicillin with purities greater than 95% wt in all runs.

 Please wait while we load your content...
Please wait while we load your content...