Theoretical study of copper complexes with lipoic and dihydrolipoic acids†
Abstract
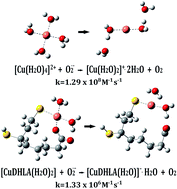
The antioxidant capacity of the deprotonated forms of lipoic and dihydrolipoic acids through their formation of complexes with copper has been theoretically studied. The relative stability of the various Cu(II) complexes considered has been studied at the M06-2X/6-31++G(d, p) level of theory combined with the SMD continuum solvation model in water under physiological pH conditions. The most stable complexes of Cu(II) are those formed with deprotonated dihydrolipoate when coordination involves the carboxylate group and one of the deprotonated thiol groups (in particular the primary one). The most thermodynamically stable Cu(II) complex was found to have antioxidant capacity, since its presence can slow down by two orders the first step of the Haber–Weiss cycle (from 1.29 × 108 M−1 s−1 to 1.33 × 106 M−1 s−1) and reduce the potential damage caused by ˙OH radical formation.


 Please wait while we load your content...
Please wait while we load your content...