Synthesis of unsaturated secondary amines by direct reductive amination of aliphatic aldehydes with nitroarenes over Au/Al2O3 catalyst in continuous flow mode†
Abstract
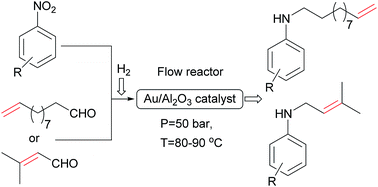
A series of unsaturated secondary amines was successfully synthesized by direct reductive amination of aliphatic aldehydes with nitroarenes over a 2.5% Au/Al2O3 catalyst in a continuous flow reactor using molecular hydrogen as a reducing agent. In most cases, the targeted secondary amines were obtained in good to excellent yields. Interestingly, the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group is practically absent in both initial aldehydes and secondary amines under the reaction conditions. It was found that the introduction of electron-donating substituents in the para- and meta-position of nitrobenzenes increased the yield of secondary amine, while in the case of nitrobenzenes with electron-withdrawing substituents or electron-donating substituents in the ortho-position a decrease in the yield of the target product was observed.
C group is practically absent in both initial aldehydes and secondary amines under the reaction conditions. It was found that the introduction of electron-donating substituents in the para- and meta-position of nitrobenzenes increased the yield of secondary amine, while in the case of nitrobenzenes with electron-withdrawing substituents or electron-donating substituents in the ortho-position a decrease in the yield of the target product was observed.


 Please wait while we load your content...
Please wait while we load your content...