X-ray crystallographic evidence for the simultaneous presence of axial and rhombic sites in cupredoxins: atomic resolution X-ray crystal structure analysis of pseudoazurin and DFT modelling†
Abstract
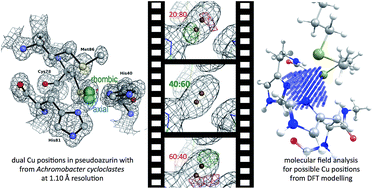
Crystal structure refinement of pseudoazurin from Achromobacter cycloclastes (AcPAz) was carried out at atomic (1.10 Å) resolution. The copper ion was localized in two positions at the metal binding site of AcPAz. The occupancies of the copper sites are consistent with the ratio of axial and rhombic signals from EPR spectra and the intensity ratios for blue (axial) and green (rhombic) copper sites from UV-VIS spectroscopy. Computational modelling using an approximately 6 Å protein environment around the Cu site for both the oxidized and reduced forms showed that a small scale inner sphere rearrangement can account for the co-existence of two different redox active sites for a mononuclear cupredoxin independently from the employed density functionals.

 Please wait while we load your content...
Please wait while we load your content...