Fluorescent oxazoles from quinones for bioimaging applications†
Abstract
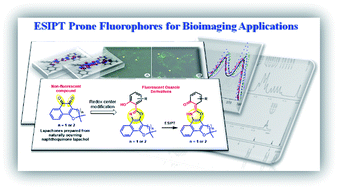
This work describes a synthetic strategy for the syntheses of four new fluorescent excited state intramolecular proton transfer (ESIPT) prone oxazole derivatives synthesized from lapachol, a naturally occurring naphthoquinone isolated from the Tabebuia species (ipe tree). DFT calculations were performed to understand the ESIPT stabilizing process of these new derivatives. The new structures were designed to have improved lipophilic and balanced hydrophobic properties toward a selective cellular staining of lipid-based structures, that is, lipid inclusions in the cytosol. Cell-imaging experiments returned interesting results and showed the molecular architecture of the four derivatives had a great influence over the stabilizing processes in the excited state and over the selection of lipid inclusions inside the cells.


 Please wait while we load your content...
Please wait while we load your content...