An iron-based micropore-enriched silica catalyst: in situ confining of Fe2O3 in the mesopores and its improved catalytic properties†
Abstract
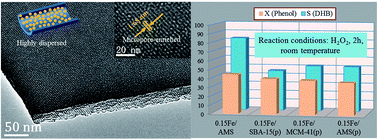
Surface exposed catalytic active species are thought to be responsible for overall catalytic activity and selectivity. In this paper, controllable contents of iron oxides were in situ introduced into the inner surface of anionic surfactant-templated mesoporous silica (AMS) by the metal-modified anionic surfactant templating route, in which anionic micelles decorated with different amounts of Fe2+ were direct utilized to assemble with organosilicate. The characterization results demonstrated that, after thermal treatments, Fe2O3 species were in situ formed and highly-dispersed in the channels of AMS, and the pore size was accordingly tailored to the microporous level (<2 nm). In each AMS catalyst, Fe2O3 showed better dispersion and stability compared with the post-impregnated method. Moreover, the catalytic performances of phenol hydroxylation on the as-synthesized catalysts were significantly enhanced, and the optimal catalyst (Fe/Si = 3.5 wt%) gave a phenol conversion of 44.3% with a selectivity of 82.6% to dihydroxybenzene. By comparison with some reference mesoporous silica catalysts (0.15Fe/MCM-41 and 0.15Fe/SBA-15), the iron-based micropore-enriched silica catalyst (Fe/AMS) gave an obviously higher selectivity to dihydroxybenzene. Finally, the optimal catalyst was examined for at least 5 runs in phenol hydroxylation.

 Please wait while we load your content...
Please wait while we load your content...