A new polymorphic form and polymorphic transformation of loratadine†
Abstract
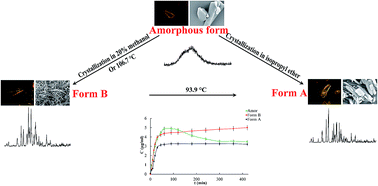
In this work, a new form of loratadine (Form B) was prepared from a 20% methanol and 80% water mixture and characterized by polarizing microscopy (POM), scanning electron microscopy (SEM), powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC). The dissolution, solubility and interfacial energy of Form B in water were measured and compared with those of amorphous and Form A loratadine. In addition, the solid-state transformations of loratadine solid forms were investigated by hot-stage microscopy (HSM) and DSC while the non-isothermal crystallization of amorphous loratadine was studied by DSC. It was found that Form B has a unique acicular structure and PXRD pattern, which are different from Form A. Moreover, Form B has a higher equilibrium solubility compared with Form A. The results of the solid-state transformation investigation (DSC) shows that the amorphous form was changed to the new polymorph B at 106.7 °C and Form B converted exothermally to Form A at 93.9 °C during heating. In the non-isothermal crystallization of amorphous loratadine, the crystallization rate constant (Zt) increased with the increasing heating rate and the Avrami exponent (n) was found to be about 3. Hopefully, Form B will be considered to be a promising approach to improve the loratadine solubility/dissolution rate.

 Please wait while we load your content...
Please wait while we load your content...