Precursor dissolution temperature as a size-controller in Fe3O4 submicrospheres syntheses and their effect in the catalytic degradation of Rhodamine B†
Abstract
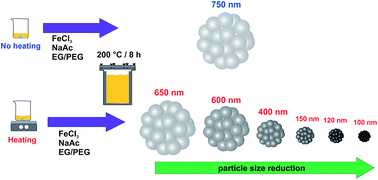
Iron oxides submicrospheres have been synthesized by a solvothermal method. The particle sizes decrease from 700 to 100 nm as a function of dissolution temperature of iron salt precursors, when keeping the reaction temperature in the autoclave constant. The submicrosphere particles are formed by aggregation of smaller nanoparticles, nanograins. The Fe3O4 submicrospheres show high saturation magnetization (Ms) and low hysteresis (low remnant magnetization, Mr and coercivity, Hc), showing superparamagnetic behavior. The size tailoring of the iron oxide particles allowed their application as catalysts on the photo-Fenton reaction of Rhodamine B degradation, in which smaller particles showed high catalytic activity and the degradation efficiency showed strong dependence on the nanograin size.

 Please wait while we load your content...
Please wait while we load your content...