Efficient confinement of ionic liquids in MIL-100(Fe) frameworks by the “impregnation-reaction-encapsulation” strategy for biodiesel production
Abstract
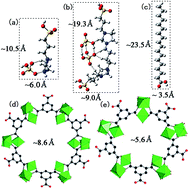
A new, simple and effective strategy to confine dicationic acid ionic liquids (DAILs, 1,4-bis[3-(propyl-3-sulfonate) imidazolium] butane hydrogen sulfate) in the cages of MIL-100(Fe) frameworks was constructed. The target catalyst, defined as MIL-100(Fe)@DAILs, was characterized by XRD, FTIR, SEM, TEM, EA, TGA and N2 adsorption–desorption. Meanwhile, the catalytic activity of the MIL-100(Fe)@DAILs catalyst was evaluated by the esterification reaction with oleic acid and methanol. The results indicated that the DAILs had been effectively encapsulated within the cages of the MIL-100(Fe) frameworks. Moreover, the influence of reaction time, reaction temperature, molar ratio of methanol to oleic acid and catalyst dosage on the conversion of oleic acid was studied by univariate analysis. The conversion of oleic acid decreased from 93.5% to 86.0% when the catalyst was reused five times, which indicated that the target catalyst possessed higher catalytic activity and superior catalytic activity. Finally, an esterification mechanism catalyzed by this novel catalyst was illustrated.

 Please wait while we load your content...
Please wait while we load your content...