Multifunctional aldose reductase inhibitors based on 2H-benzothiazine 1,1-dioxide†
Abstract
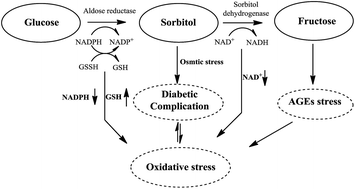
A series of benzothiazine derivatives were designed and synthesized for the development of drug candidates for diabetic complications. A number of derivatives having a phenolic hydroxyl-substituted N2-aromatic side chain and a C4-acetic acid head group on the core structure were found to be potent and selective aldose reductase inhibitors. 8a with a phenolic 4-hydroxyl at N2-styryl side chain was proved to be the most active with an IC50 value of 0.094 μM. All compounds with the N2-styryl side chain showed good antioxidant activity using the DPPH radical scavenging test, and among them, compounds with phenolic hydroxyl-substituted N2-styryl were potent both in activities of ALR2 inhibition and antioxidation. The results suggest a success in the development of multifunctional aldose reductase inhibitors based on the benzothiazine framework.

 Please wait while we load your content...
Please wait while we load your content...