A highly efficient potassium-treated Au–Cu/Al2O3 catalyst for the preferential oxidation of carbon monoxide†
Abstract
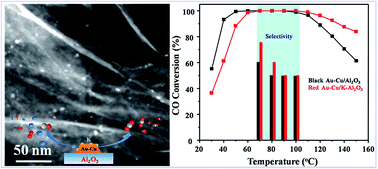
At the operating temperature (80–120 °C) of a proton exchange membrane fuel cell (PEMFC), high-efficiency elimination of CO while minimizing the H2 consumption processes is highly desired but still remains a challenge. In the present manuscript, one novel potassium-treated Au–Cu/Al2O3 catalyst was synthesized via a two step deposition–precipitation (DP) method with excellent catalytic performance for preferential oxidation of CO (CO-PROX) in a H2-rich stream. This catalyst exhibits 100% CO conversion over a wide temperature window of 60–110 °C and ≥50% selectivity of CO2 under the PEMFC operating temperature. Furthermore, the as-prepared potassium-treated Au–Cu/Al2O3 catalysts were also characterized by N2 adsorption analysis, scanning transmission electron microscopy (STEM)-energy dispersive X-ray spectroscopy (EDX), and in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS), and the reasons for enhanced catalytic activity of the potassium-treated sample were elucidated. The introduction of copper could strengthen the CO adsorption on the Au–Cu/Al2O3 catalyst and potassium treatment could significantly increase the stability of active Cu+ species that contribute to enhanced catalytic performance.

 Please wait while we load your content...
Please wait while we load your content...