Back to basics: identification of reaction intermediates in the mechanism of a classic ligand substitution reaction on Vaska's complex†
Abstract
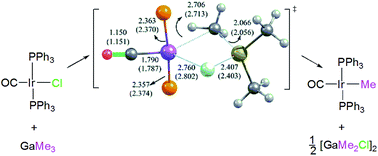
The mechanism of methylation of Vaska's complex trans-[ClIr(CO)(PPh3)2] by trimethylgallium was studied and the identification of the spectroscopically detected intermediates was achieved with the aid of computational methods. The reaction pathway, computed by means of density functional theory (M05-2X-D3/def2-SVP), involves the initial formation of a chloride-bridged adduct trans-[(Cl·GaMe3)Ir(CO)(PPh3)2] to then proceeds to a transition state [(μ2-Cl,C-ClMeGaMe2)Ir(CO)(PPh3)2]. This transition state subsequently evolves to the methylated adduct [MeIr(CO)(PPh3)2·(GaMe2Cl)] to finally release the alkylated product trans-[MeIr(CO)(PPh3)2] together with GaMe2Cl.

 Please wait while we load your content...
Please wait while we load your content...