Rh-catalysed asymmetric conjugate addition of boronic acids to nitroalkenes employing a P-chiral P,π-hybrid ligand†
Abstract
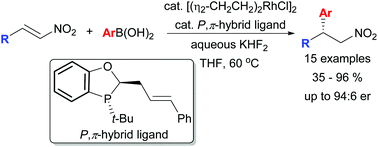
A Rh-catalysed asymmetric conjugate addition of aryl boronic acids to β-substituted nitroalkenes was developed employing a P-chiral P-alkene hybrid bidentate ligand with enantioselectivities of up to 94 : 6 er. DFT modelling of the transition state for the addition reaction was consistent with our previous model for stereocontrol employing this family of chiral ligands. Application of the β-chiral nitroalkanes was demonstrated in the intramolecular Buchwald–Hartwig amination and aminocarbonylation to provide 5- and 6-membered chiral heterocycles.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...