Equilibrium acidities of BINOL type chiral phenolic hydrogen bonding donors in DMSO†
Abstract
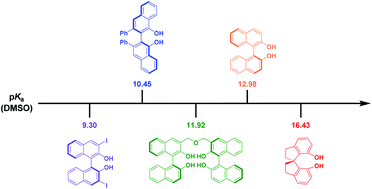
The pKa values of fifteen BINOL type chiral phenolic catalysts were determined by the overlapping indicator method in DMSO via UV spectrophotometric titrations. The acidities cover the range from 9.30 to 16.43. The pKa gap between BINOL and 2-naphthol was explained by IR spectrum data, crystal structure and 1H NMR. The relationship between the pKa values of 3,3′-modified BINOLs and Hammett ortho substituent constants (σo−) was investigated and a good correlation (R2 = 0.984) was obtained. The results may be helpful for the rational design and development of novel BINOL type phenolic catalysts, sensors and ligands.

 Please wait while we load your content...
Please wait while we load your content...