N-Ferrocenylsulfonyl-2-methylaziridine: the first ferrocene monomer for the anionic (co)polymerization of aziridines†
Abstract
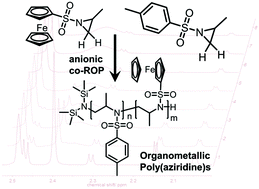
N-Ferrocenylsulfonyl-2-methylaziridine (fcMAz) is synthesized in a convenient three-step protocol from ferrocene. It represents the first aziridine-based monomer suitable for the anionic polymerization, carrying a ferrocenyl-substituent. Sulfonamide-activated aziridines can undergo (mostly living) anionic polymerization and fcMAz is also the first example of a functional sulfonamide-based aziridine monomer. It can be polymerized with different initiators to homo and copolymers (block or statistical) with adjustable molecular weights. The homopolymer is insoluble in common organic solvents, while the copolymers with other aziridines are soluble and exhibit molecular weight dispersities of 1.1–1.5. FcMAz thus broadens the scope of ferrocenyl-containing polymers and may be combined with other anionic polymerization techniques in the future.


 Please wait while we load your content...
Please wait while we load your content...