Well-defined polymers containing a single mid-chain viologen group: synthesis, environment-sensitive fluorescence, and redox activity†
Abstract
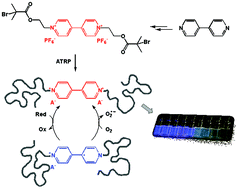
A difunctional viologen-based alkyl halide initiator was synthesized and used to initiate the copper-mediated atom transfer radical polymerization (ATRP) of methyl methacrylate to afford well-defined polymers with a single backbone viologen functionality situated in the middle of the chain. Conducting low-catalyst-concentration ATRP in the presence of reducing agents was challenging due to the occurrence of side redox reactions between the viologen and the highly reducing CuI-based ATRP activator (derived from tris(2-pyridylmethyl)amine), as well as between the produced viologen radical-cations and the primary and/or propagating radicals. However, when the less-reducing CuI complex of 2,2′-bipyridine was employed as the ATRP activator in the absence of external reducing agents, the side reactions were suppressed and controlled polymerizations took place. The polymers with the internal viologen group were fluorescent, and both the intensity and the color of the emitted light depended on the nature of the solvent. The fluorescence was quenched in the presence of polarizable anions (chloride, bromide, iodide, and thiocyanate), and nitrobenzene, even at relatively low (μM) concentrations. The viologen-containing polymers were also used as redox catalysts for the oxidation of phenylhydrazine by oxygen from air.

 Please wait while we load your content...
Please wait while we load your content...