Bicyclic isoureas derived from 1-deoxynojirimycin are potent inhibitors of β-glucocerebrosidase†
Abstract
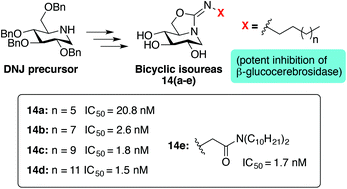
A series of bicyclic isourea derivatives were prepared from 1-deoxynojirimycin using a concise synthetic protocol proceeding via a guanidino intermediate. Inhibition assays with a panel of glycosidases revealed that these deoxynojirimycin-derived bicyclic isoureas display very potent inhibition against human recombinant β-glucocerebrosidase with IC50 values in the low nanomolar range.

 Please wait while we load your content...
Please wait while we load your content...