Reactions of 1,2-diaza-1,3-butadienes with propargyl alcohol as an approach to novel bi-heterocyclic systems†
Abstract
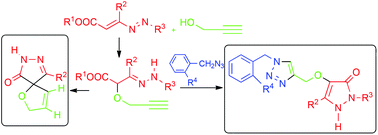
Here we describe the reaction of 1,2-diaza-1,3-dienes and propargyl alcohol furnishing α-(prop-2-yn-1-yloxy)hydrazones that are converted into novel alkyl-1-oxa-7,8-diazaspiro[4.4]nona-3,8-dien-6-ones, by means of 2,3-Wittig rearrangement under very mild conditions. The same α-(prop-2-yn-1-yloxy)hydrazones, treated with benzyl azides furnish the corresponding α-[(1,2,3-triazol-4-yl)methoxy]hydrazones, via Cu(II)-catalyzed 1,3-dipolar cycloaddition. Their subsequent base-promoted cyclization produces interesting pyrazolone–triazole derivatives. The impact of this work can be ascribable to the attractiveness of the bi-heterocyclic systems obtained and to the ease of the synthetic methodologies proposed.

 Please wait while we load your content...
Please wait while we load your content...