A step-economical multicomponent synthesis of 3D-shaped aza-diketopiperazines and their drug-like chemical space analysis†
Abstract
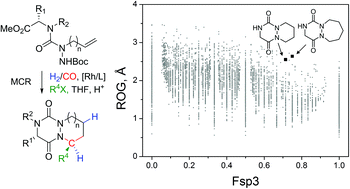
A rapid and atom economical multicomponent synthesis of complex aza-diketopiperazines (aza-DKPs) driven by Rh(I)-catalyzed hydroformylation of alkenylsemicarbazides is described. Combined with catalytic amounts of acid and the presence of nucleophilic species, this unprecedented multicomponent reaction (MCR) enabled the formation of six bonds and a controlled stereocenter from simple substrates. The efficacy of the strategy was demonstrated with a series of various allyl-substituted semicarbazides and nucleophiles leading to the preparation of 3D-shaped bicyclic aza-DKPs. Moreover, an analysis of their 3D molecular descriptors and “drug-likeness” properties highlights not only their originality in the chemical space of aza-heterocycles but also their great potential for medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...