Ethionamide biomimetic activation and an unprecedented mechanism for its conversion into active and non-active metabolites†
Abstract
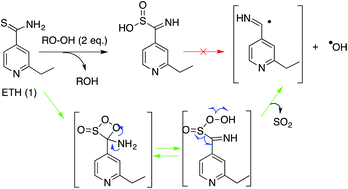
Ethionamide (ETH), a second-line anti-tubercular drug that is regaining a lot of interest due to the increasing cases of drug-resistant tuberculosis, is a pro-drug that requires an enzymatic activation step to become active and to exert its therapeutic effect. The enzyme responsible for ETH bioactivation in Mycobacterium tuberculosis is a monooxygenase (EthA) that uses flavin adenine dinucleotide (FAD) as a cofactor and is NADPH- and O2-dependant to exert its catalytic activity. In this work, we investigated the activation of ETH by various oxygen-donor oxidants and the first biomimetic ETH activation methods were developed (KHSO5, H2O2, and m-CPBA). These simple oxidative systems, in the presence of ETH and NAD+, allowed the production of short-lived radical species and the first non-enzymatic formation of active and non-active ETH metabolites. The intermediates and the final compounds of the activation pathway were well characterized. Based on these results, we postulated a consistent mechanism for ETH activation, not involving sulfinic acid as a precursor of the iminoyl radical, as proposed so far, but putting forward a novel reactivity for the S-oxide ethionamide intermediate. We proposed that ETH is first oxidized into S-oxide ethionamide, which then behaves as a “ketene-like” compound via a formal [2 + 2] cycloaddition reaction with peroxide to give a dioxetane intermediate. This unstable 4-membered intermediate in equilibrium with its open tautomeric form decomposes through different pathways, which would explain the formation of the iminoyl radical and also that of different metabolites observed for ETH oxidation, including the ETH–NAD active adduct. The elucidation of this unprecedented ETH activation mechanism was supported by the application of isotopic labelling experiments.

 Please wait while we load your content...
Please wait while we load your content...