Platinum-catalyzed cycloisomerizations of a common enyne: a divergent entry to cyclopropane sesquiterpenoids. Formal synthesis of sarcandralactone A†
Abstract
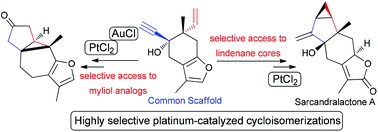
A common enyne scaffold, resembling the structures of natural elemanes was found to be an excellent substrate for highly regioselective cycloisomerizations to produce diverse cyclopropane sesquiterpenoids. Platinum-catalysis was utilized to produce either lindenane or myliol cores, found in natural products, starting from enyne acetate 10 and its corresponding allene 12 respectively. Based on this concept, a second generation strategy allows the formal synthesis of sarcandralactone A.

 Please wait while we load your content...
Please wait while we load your content...