Arginine side-chain modification that occurs during copper-catalysed azide–alkyne click reactions resembles an advanced glycation end product†
Abstract
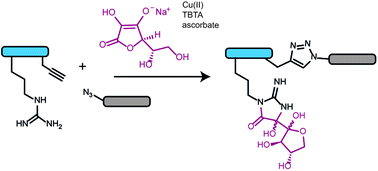
Dehydroascorbate is a by-product of copper-catalysed azide–alkyne click (CuAAC) reactions and also forms advanced glycation end products (AGEs) in tissues undergoing oxidative stress. Here we isolate and characterize an arginine–dehydroascorbate adduct formed during CuAAC reactions, investigate strategies for preventing its formation, and propose its biological relevance as an AGE.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...