Regioselective carboannulation of electron-deficient allenes with dialkyl (2-formylphenyl)malonates leading to multisubstituted naphthalenes†
Abstract
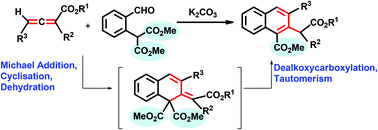
An efficient base-catalysed regioselective carboannulation of allenoates (or allenylphosphonates) with dialkyl 2-(2-formylphenyl)malonates that leads to multi-substituted naphthalenes in high yields has been developed. This cascade reaction proceeds through Michael addition, cyclisation, dealkoxycarboxylation and tautomerisation. By using an allenylphosphine oxide, a species analogous to one of the intermediate species in the mechanistic pathway has been isolated.

 Please wait while we load your content...
Please wait while we load your content...