The cooperative FeCl3/DDQ system for the regioselective synthesis of 3-arylindoles from β-monosubstituted 2-alkenylanilines†
Abstract
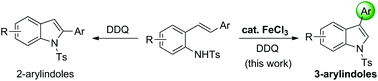
A highly regioselective synthesis of 3-arylindoles by using the cooperative FeCl3/DDQ system has been developed. This new protocol represents an attractive route for the synthesis of 3-arylindoles from readily accessible non-indole precursors, β-aryl-substituted 2-styrylanilines, using an inexpensive catalyst and oxidant. Noteworthy is the unique synergetic and synergistic effect of FeCl3 and DDQ on the 1,2-aryl migratory process.

 Please wait while we load your content...
Please wait while we load your content...