Formation of DPM ethers using O-diphenylmethyl trichloroacetimidate under thermal conditions†
Abstract
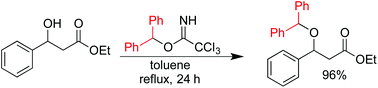
Alcohols are effectively converted to their corresponding diphenylmethyl (DPM) ethers by reaction with O-diphenylmethyl trichloroacetimidate in refluxing toluene without the requirement of a catalyst or other additives. A number of acid and base sensitive substrates were protected in excellent yield using this new method without disturbing the pre-existing functionality present in these molecules. This reaction is the first example of the formation of an ether from stoichiometric amounts of a trichloroacetimidate and an alcohol without the addition of a Brønsted or Lewis acid catalyst.

 Please wait while we load your content...
Please wait while we load your content...