Design, synthesis and in vitro evaluation of d-glucose-based cationic glycolipids for gene delivery†
Abstract
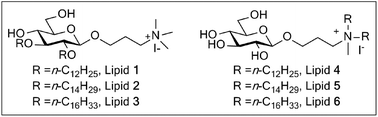
A cationic lipid consists of a hydrophilic headgroup, backbone and hydrophobic tails which have an immense influence on the transfection efficiency of the lipid. In this paper, two novel series of cationic cyclic glycolipids with a quaternary ammonium headgroup and different-length hydrophobic tails (dodecyl, tetradecyl, hexadecyl) have been designed and synthesized for gene delivery. One contains lipids 1–3 with two hydrophobic alkyl chains linked to the glucose ring directly via an ether link. The other contains lipids 4–6 with two hydrophobic chains on the positively charged nitrogen atoms. All of the lipids were characterized for their ability to bind to DNA, size, ζ-potential, and toxicity. Atomic force microscopy showed that the lipids and DNA–lipid complexes were sphere-like forms. The lipids were used to transfer enhanced green fluorescent protein (EGFP-C3) to HEK293 cells without a helper lipid, the results indicated that lipids 4–6 have better transfection efficiency, in particular lipids 5–6 have similar or better efficiency, compared with the commercial transfection reagent lipofectamine 2000.

 Please wait while we load your content...
Please wait while we load your content...