Carbon-rich cyclopentadienyl ruthenium allenylidene complexes†
Abstract
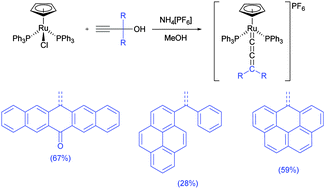
Ruthenium allenylidene complexes with carbon-rich polyaromatic moieties have been synthesized by using [RuCl(η5-C5H5)(PPh3)2] (η5-C5H5 = cyclopentadienyl) as a precursor and the propargyl alcohols 10-ethynyl-10-hydroxyanthracen-9-one (ACO), 13-ethynyl-13-hydroxypentacen-6-one (PCO), 1-phenyl-1-(pyren-1-yl)prop-2-yn-1-ol (PyrPh), 9-ethynyl-9H-fluoren-9-ol (FN) and 6-ethynyl-6H-benzo[cd]pyren-6-ol (BPyr) as ligands. The resulting cationic allenylidene complexes, [Ru(η5-C5H5)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (AO))(PPh3)2]PF6 (1), [Ru(η5-C5H5)(
(AO))(PPh3)2]PF6 (1), [Ru(η5-C5H5)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (PCO))(PPh3)2]PF6 (2), [Ru(η5-C5H5)(
(PCO))(PPh3)2]PF6 (2), [Ru(η5-C5H5)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (PyrPh))(PPh3)2]PF6 (3), [Ru(η5-C5H5)(
(PyrPh))(PPh3)2]PF6 (3), [Ru(η5-C5H5)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (FN))(PPh3)2]PF6 (4), and [Ru(η5-C5H5)(
(FN))(PPh3)2]PF6 (4), and [Ru(η5-C5H5)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (BPyr))(PPh3)2]PF6 (5) show interesting intermolecular π-interactions in the solid-state structure as well as solution state complexation with pyrene (documented by Job's plots experiments). CV data indicate possible Ru(II)/Ru(III) oxidation, as well as the potential reduction of the carbon-rich allenylidene moiety.
(BPyr))(PPh3)2]PF6 (5) show interesting intermolecular π-interactions in the solid-state structure as well as solution state complexation with pyrene (documented by Job's plots experiments). CV data indicate possible Ru(II)/Ru(III) oxidation, as well as the potential reduction of the carbon-rich allenylidene moiety.

 Please wait while we load your content...
Please wait while we load your content...