Syntheses of polycyclic aromatic diimides via intramolecular cyclization of maleic acid derivatives†
Abstract
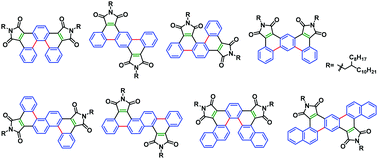
Using readily available aryl glyoxylic acids and arylene diacetic acids as starting materials, a series of polycyclic aromatic molecules bearing two phthalimide functional groups are synthesized via Perkin condensation followed by intramolecular cyclization reactions. Two different cyclization methods, photo-oxidation and Heck cross-coupling, are employed, both of which effectively accomplish the transformations from diaryl maleic anhydride or maleimide to polycyclic aromatic phthalimide functionality. The photocyclization protocol conveniently allows direct bridging of two plain aromatic C–H sites linked by a maleic anhydride group and uniquely produces the more twisted polycyclic framework as the major product, whereas the Heck coupling approach can typically afford more extended polycyclic skeletons. Thionation reactions are then carried out for the obtained polycyclic diimide molecules using Lawesson's reagent. For all isolated stable products, partial thionation occurs. The prepared polycyclic diimide compounds possess relatively low LUMO levels, and thionation further decreases the LUMO energy of the molecules by 0.2–0.3 eV. Electron-transporting properties are characterized by using solution-processed OFET devices, and an electron mobility of 0.054 cm2 V−1 s−1 is demonstrated by a selected compound. Such semiconducting performance promises great potentials of this class of compounds as useful electron-accepting and transporting building blocks in developing various new semiconductive materials.

 Please wait while we load your content...
Please wait while we load your content...