Synthesis and biological evaluation of novel indole derivatives containing sulfonamide scaffold as potential tubulin inhibitor†‡
Abstract
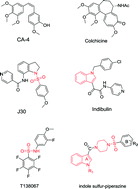
Microtubule-targeted drugs play a critical role in various types of cancer therapy worldwide. In our study, a series of novel indole derivatives containing a sulfonamide scaffold were designed, synthesized and biologically evaluated as potential tubulin polymerization inhibitors. Among them, compound 18 displayed the most potent inhibitory effect on antiproliferative activity against four types of human cancer cell lines (IC50 values of 0.24–0.59 μM) and tubulin assembly (IC50 = 1.82 μM). Further investigation demonstrated that compound 18 is a potent inducer of apoptosis in HeLa cells and could cause cell arrest in the G2/M phase of the cell cycle. Molecular docking and confocal microscopy assay results indicate that compound 18 could bind tightly to the colchicine binding site of tubulin and act as a tubulin polymerization inhibitor. A 3D-QSAR model was also built to provide more information which could be applied to the design of new molecules with more potent tubulin inhibitory activity.

 Please wait while we load your content...
Please wait while we load your content...