Abstract
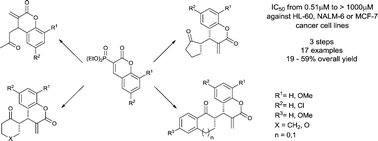
A simple and general strategy for the synthesis of 3-methylene-4-(2-oxoalkyl)-3,4-dihydrocoumarins has been developed. The elaborated synthetic protocol includes 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)- or Cs2CO3-promoted conjugate addition of enolizable ketones to 3-(diethoxyphosphoryl)coumarins followed by Horner–Wadsworth–Emmons reaction of the resulting 3-diethoxyphosphoryl-4-(2-oxoalkyl)-3,4-dihydrocoumarins with formaldehyde. For prochiral ketones, the corresponding α-methylene-δ-lactones were obtained as single syn-diastereoisomers. All α-methylene-δ-lactones were evaluated in vitro for their cytotoxic activity against two human leukemia cell lines, HL-60 and NALM-6, as well as the MCF-7 breast cancer cell line. One selected compound, 13h, was further tested in order to elucidate the mechanism behind its cytotoxic effect. In MCF-7 cells, this compound was shown to induce subG0/G1 cell cycle arrest, increased Bax and decreased Bcl-2 mRNA levels, and cause the dissipation of the mitochondrial membrane potential which indicated that it induced the intrinsic pathway of apoptosis.

 Please wait while we load your content...
Please wait while we load your content...