Electrochemically catalyzed amino-oxygenation of styrenes: n-Bu4NI induced C–N followed by a C–O bond formation cascade for the synthesis of indolines†
Abstract
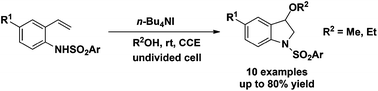
Efficient electrochemical amino-oxygenation of styrenes has been developed for the synthesis of 3-methoxyindolines and 3-ethoxyindoline, using a simple beaker-type undivided cell with n-Bu4NI serving as a redox catalyst under constant current electrolysis (CCE) conditions. The chemistry proceeds in a paired electrolysis mode, avoiding the utilization of external oxidants and bases and therefore represents an environmentally benign means. The process also works in the absence of an additional conducting salt. Gram-scale reaction further demonstrates the practicability of the protocol. Proton NMR spectroscopy was used to demonstrate that the amino-oxygenation of N-(2-vinylphenyl)sulfonamides likely involves the initial iodoamination of the alkene, followed by nucleophilic substitution of the methoxide/ethoxide generated at the cathode.

 Please wait while we load your content...
Please wait while we load your content...