Oxidative conversion of glucose to gluconic acid by iron(iii) chloride in water under mild conditions†
Abstract
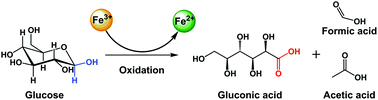
A simple method was demonstrated to oxidize glucose into gluconic acid in a concentrated FeCl3 solution. The maximum gluconic acid yield (52.3%) was achieved in the 40% FeCl3 solution at 110 °C in 4 hours. Formic and acetic acids were the main coproducts with an yield of 10–20%.

 Please wait while we load your content...
Please wait while we load your content...