Improved photocatalytic hydrogen evolution driven by chloro(terpyridine)platinum(ii) derivatives tethered to a single pendant viologen acceptor†
Abstract
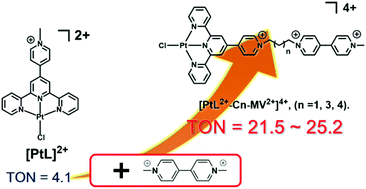
Three chloro(4′-(N-methylpyridinium)-2,2′:6′,2′′-terpyridine)platinum(II) (abbreviated as PtL2+) derivatives tethered to a single alkyl viologen unit (–(CH2)n–CH2–N+C5H4–C5H4N+–CH3; abbreviated as -Cn-MV2+, where n = 1, 3, and 4), i.e., PtL2+-Cn-MV2+, have been synthesized and investigated in detail. It is shown that the turnover number (TON) for the photocatalytic H2 evolution from water in the presence of a sacrificial electron donor EDTA (ethylenediaminetetraacetic acid disodium salt) is dramatically improved by the attachment of a single alkyl MV2+ unit (TON = 21.5–25.2, 12 h). Spectrophotometric studies reveal that the photoirradiation of PtL2+-Cn-MV2+ in the presence of EDTA initially leads to the formation of a 1-electron-reduced species, and then to a 2-electron-reduced species, where reductive quenching of a photoexcited species is a major path to the reduced photoproduct in each step. Electrochemical studies show that two consecutive 1-electron reductions at the PtL2+ unit are nearly overlapped with the corresponding reductions at the MV2+ unit. The 1-electron-reduced species can be thus expressed as either PtL+˙-Cn-MV2+ or PtL2+-Cn-MV+˙, while the 2-electron-reduced one as PtL+˙-Cn-MV+˙. Moreover, the latter products behave as stacked species involving three types of π-dimer sites, (PtL+)2, (MV+)2, and (PtL+)(MV+), and do not drive thermal H2 evolution according to the reaction: PtL+˙-Cn-MV+˙ + 2H+ → PtL2+-Cn-MV2+ + H2. The H2 evolution from water photocatalyzed by PtL2+-Cn-MV2+ has been found to occur via formation of 3-electron-reduced species; PtL+˙-Cn-MV+˙ + EDTA + hν → PtL0-Cn-MV+˙ (or PtL+˙-Cn-MV0) + EDTA(ox), and PtL0-Cn-MV+˙ (or PtL+˙-Cn-MV0) + 2H+ → PtL+˙-Cn-MV2+ (or PtL2+-Cn-MV+˙) + H2.

 Please wait while we load your content...
Please wait while we load your content...