Silver(i) complexes with 1′-(diphenylphosphino)-1-cyanoferrocene: the art of improvisation in coordination†
Abstract
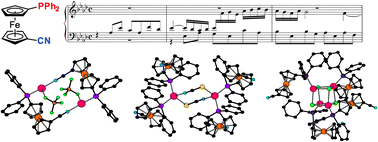
1′-(Diphenylphosphino)-1-cyanoferrocene (1) reacts with silver(I) halides at a 1 : 1 metal-to-ligand ratio to afford the heterocubane complexes [Ag(μ3-X)(1-κP)]4, where X = Cl (2), Br (4), and I (5). In addition, the reaction with AgCl with 2 equiv. of 1 leads to chloride-bridged dimer [(μ-Cl)2{Ag(1-κP)2}2] (3) and, presumably, also to [(μ(P,N)-1){AgCl(1-κP)}]2 (3′). While similar reactions with AgCN furnished only the insoluble coordination polymer [(1-κP)2Ag(NC)Ag(CN)]n (6), those with AgSCN afforded the heterocubane [Ag(1-κP)(μ-SCN-S,S,N)]4 (7) and the thiocyanato-bridged disilver(I) complex [Ag(1-κP)2(μ-SCN-S,N)]2 (8), thereby resembling reactions in the AgCl–1 system. Attempted reactions with AgF led to ill-defined products, among which [Ag(1-κP)2(μ-HF2)]2 (9) and [(μ-SiF6){Ag(1-κP)2}2] (10) could be identified. The latter compound was prepared also from Ag2[SiF6] and 1. Reactions between 1 and AgClO4 or Ag[BF4] afforded disilver complexes [(μ(P,N)-1)Ag(ClO4-κO)]2 (11) and [(μ(P,N)-1)Ag(BF4-κF)]2 (12) featuring pseudolinear Ag(I) centers that are weakly coordinated by the counter anions. A similar reaction with Ag[SbF6] followed by crystallization from ethyl acetate produced an analogous complex, albeit with coordinated solvent, [(μ(P,N)-1)Ag(AcOEt-κO)]2[SbF6]2 (13). Ultimately, a compound devoid of any additional ligands at the Ag(I) centers, [(μ(P,N)-1)Ag]2[B(C6H3(CF3)2-3,5)4]2 (14), was obtained from the reaction of 1 with silver(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate. The reaction of Ag[BF4] with two equivalents of 1 produced unique coordination polymer [Ag(1-κP)(μ(P,N)-1)]n[BF4]n (15), the structure of which contained one of the phosphinoferrocene ligands coordinated as a P,N-chelate and the other forming a bridge to an adjacent Ag(I) center. All of these compounds were structurally characterized by single-crystal X-ray crystallography, revealing that the lengths of the bonds between silver and its anionic ligand(s) typically exceed the sum of the respective covalent radii, which is in line with the results of theoretical calculations at the density-functional theory (DFT) level, suggesting that standard covalent dative bonds are formed between silver and phosphorus (soft acid/soft base interactions) while the interactions between silver and the ligand's nitrile group (if coordinated) or the supporting anion are of predominantly electrostatic nature.

 Please wait while we load your content...
Please wait while we load your content...